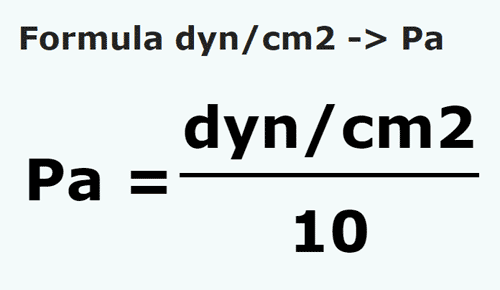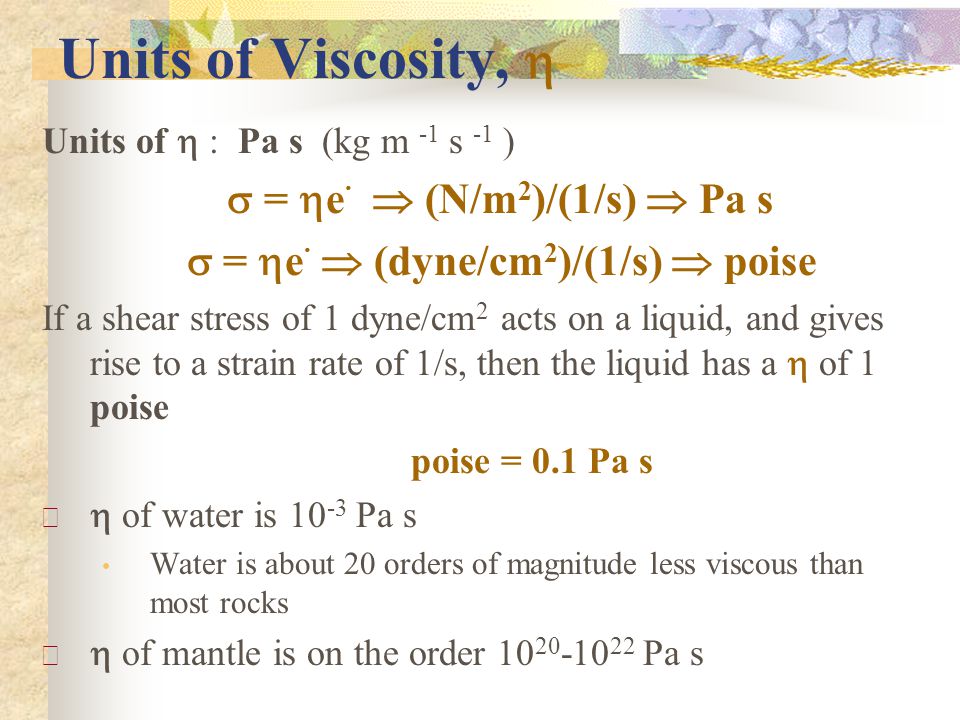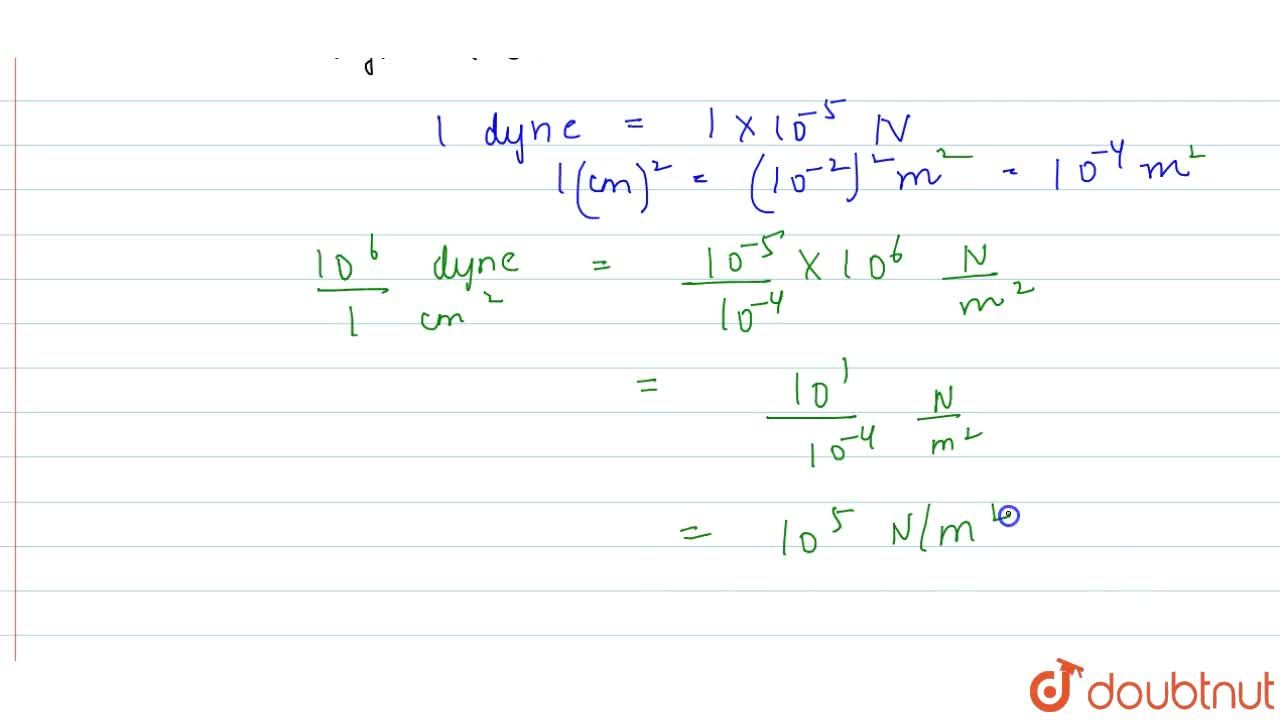Vacuum increases the mean-free-path of gas molecules.mean-free-path Vacuum prevents chemical reaction. Vacuum removes contaminants from surfaces.contaminants. - ppt download

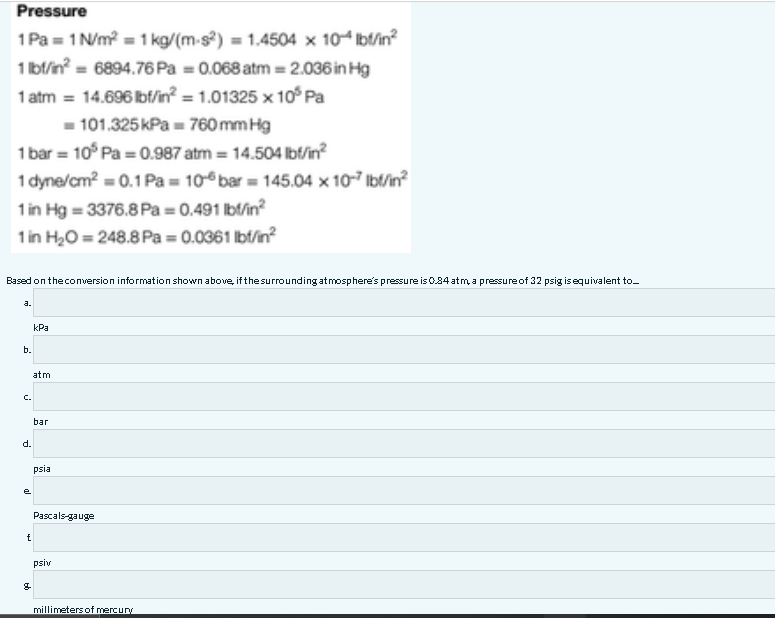
PDF) FACTORS FOR UNIT CONVERSIONS Quantity Equivalent Values | Jonathan Calvo Trenado - Academia.edu

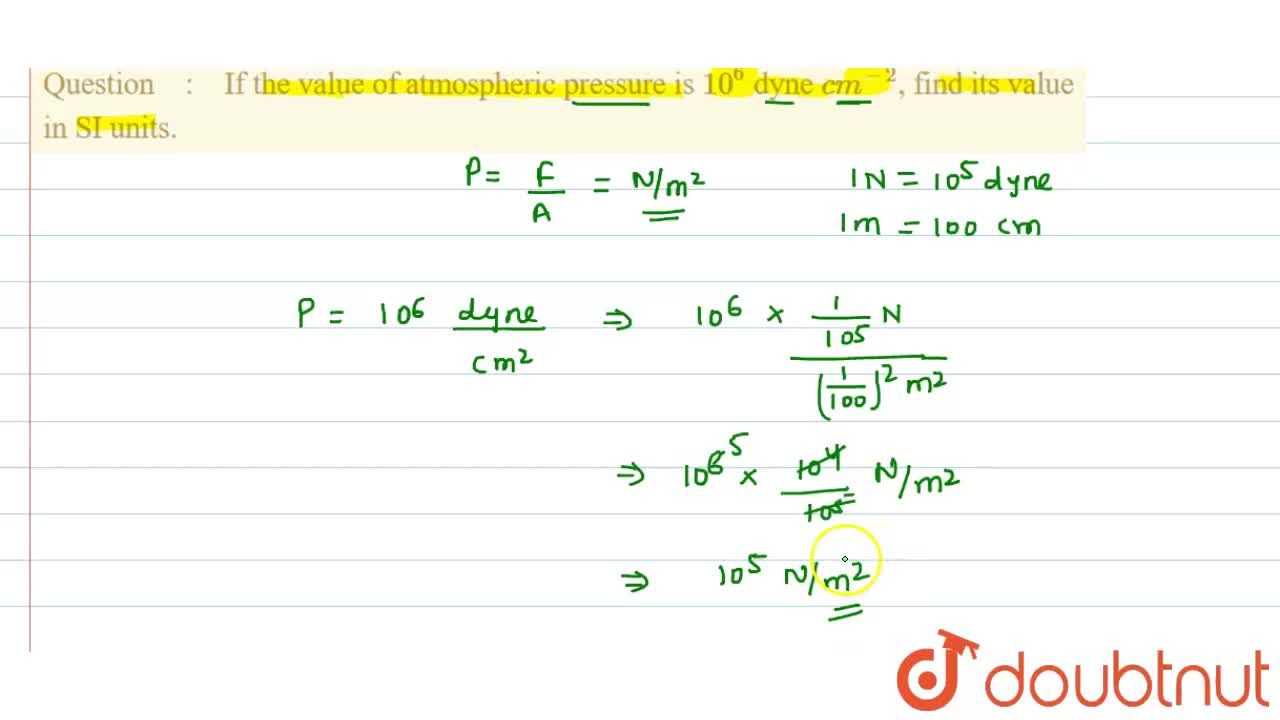
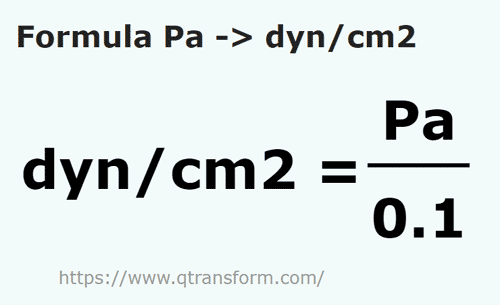
100 Pa in C G S system No links please - Physics - Units And Measurements - 14383429 | Meritnation.com

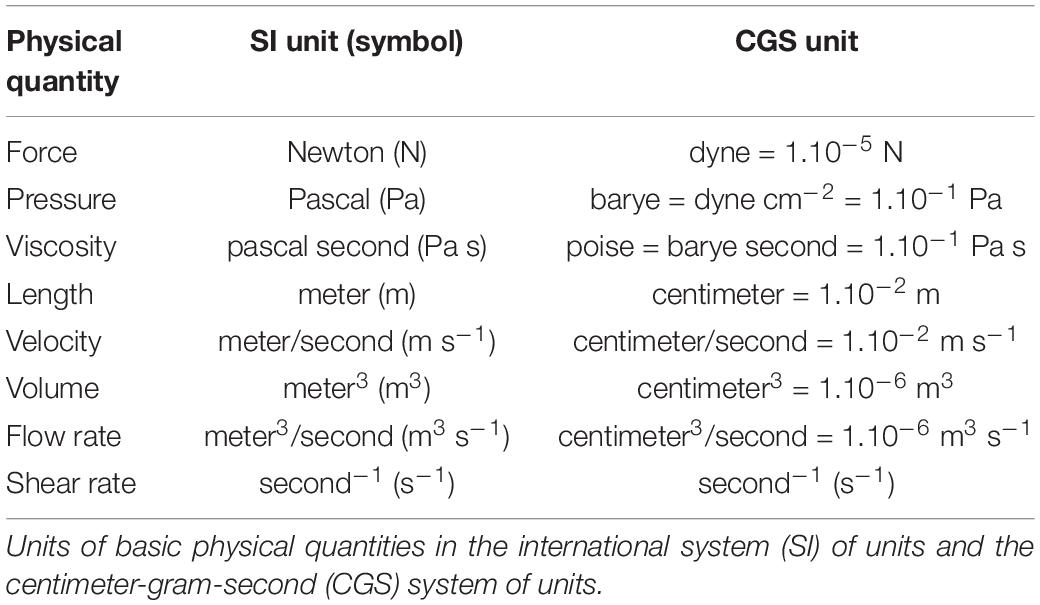
a) Estimation of wall shear stress (dynes/cm 2 ) for the same data set... | Download Scientific Diagram